St. Vincent’s Chilton receives COVID-19 vaccine
Published 1:52 pm Tuesday, December 29, 2020
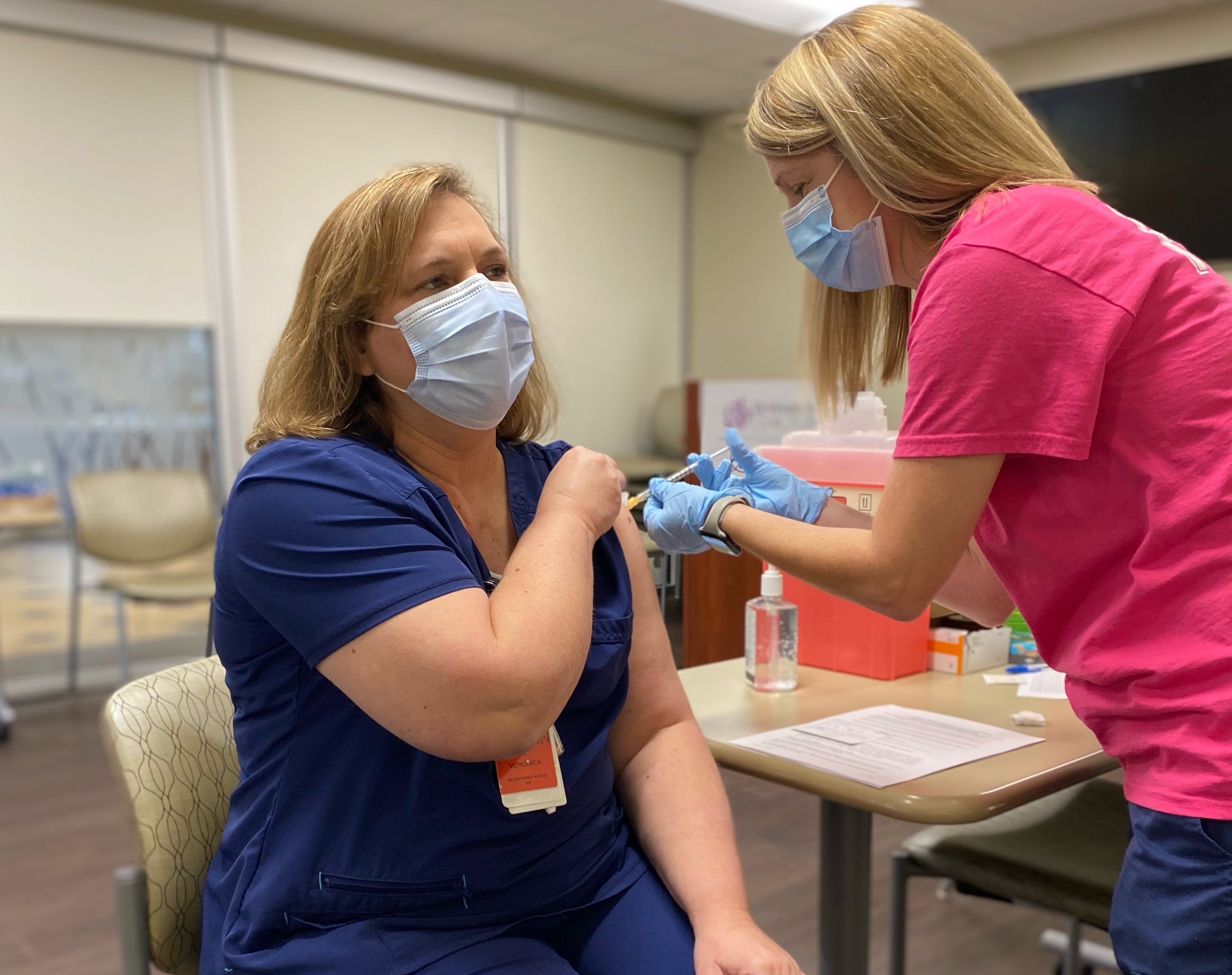
- Veronica Edwards, RN is the first Ascension St. Vincent's Chilton associate to receive the COVID-19 vaccine. (ASCENSION)
|
Getting your Trinity Audio player ready...
|
From Ascension
Associates at Ascension St. Vincent’s Chilton, which is part of Ascension St. Vincent’s, began to receive the COVID-19 vaccination the week of Christmas. The first associate to receive a vaccine was Veronica Edwards, RN, an emergency room nurse at Ascension St. Vincent’s Chilton.
“Working on the front lines, I have witnessed first-hand the strain that this pandemic has placed on hospitals, co-workers and families,” Edwards said. “I have researched the vaccine and feel knowledgeable of my decision to be vaccinated. I feel this will be beneficial in protecting my family and patients.”
Vaccines from Pfizer and Moderna have demonstrated safety and effectiveness, and the U.S. Food and Drug Administration (FDA) has authorized both vaccines for emergency use. Ascension St. Vincent’s is implementing a comprehensive vaccine distribution plan that is consistent with federal, state and local guidance.
In accordance with these guidelines, among the first group eligible to receive the vaccines are front-line caregivers – particularly those serving in emergency departments, COVID-19 units and intensive care units. For Ascension St. Vincent’s, this includes both associates and affiliated physicians and providers. Ascension St. Vincent’s anticipates the remainder of its associates will be eligible for the vaccine as more doses become available and the distribution process progresses.
Earlier this year, Ascension developed a workgroup to establish an overarching framework for equitable allocation of COVID-19 vaccines for internal and external distribution, acknowledging the need for a coordinated response by our ministry that is guided by our mission and informed by our experience of caring for the communities we serve.
As vaccine availability increases, Ascension St. Vincent’s will implement prioritization recommendations for distribution of the COVID-19 vaccine, guided by Ascension’s Mission, Vision, Values; Catholic Health Association guidance “Vaccine Equity and Catholic Principles for the Common Good”; guidance from state and federal entities; and available Centers for Disease Control and Prevention (CDC) guidance and evidence-based data on disease risk and burden.
“We strongly encourage all our associates to receive a COVID-19 vaccine when it is made available to them,” said Joseph Impicciche, President and CEO, Ascension. “In our view, this is the right thing to do to protect our associates and those we are privileged to serve. We are thankful for all who have made this vaccine possible and for the continued dedication of our selfless caregivers.”
All approved vaccines require extensive research, documentation and closely monitored clinical trials to determine effectiveness and safety before being submitted by pharmaceutical companies for approval. Ascension St. Vincent’s has been involved in one of these clinical studies, with Ascension St. Vincent’s Birmingham participating in trials for the COVID-19 vaccine developed by Moderna.
Many healthcare workers and first responders are receiving the earliest wave of available vaccines, as these professions are exposed to COVID-19 at higher rates. Residents of long-term care facilities and those with high-risk health conditions also are slated to receive vaccines early, per guidelines from the CDC.
Ascension St. Vincent’s hospitals and emergency rooms remain well prepared to safely care for people with symptoms of a heart attack, stroke, respiratory distress, emergent mental health concerns, or other serious illness or injury.






